Abstract
Purpose: Rituximab (R) use can attenuate the risks of graft-versus-host disease (GVHD) after non-myeloablative allogeneic (NMA) alloSCT. There is a paucity of information on the impact of this strategy in patients receiving myeloablative (MA) conditioning.
Experimental design: We compared the risk of GVHD after an NMA regimen of BFR (bendamustine, fludarabine, rituximab) (Khouri et al. Blood 20014;124:2306-12.) to the MA regimen of bortezomib-R-BEAM (carmustine, etoposide, cytarabine, melphalan) regimen in patients with relapsed lymphoma undergoing alloSCT. Patients were treated on 2 prospective trials at our center between 2009-2013 and 2007-2011, respectively. Patients were considered for NMA if they were not eligible for myeloablative chemotherapy. Primary GVHD prophylaxis consisted of tacrolimus and methotrexate. All patients received rituximab (375 mg/m2 intravenously on day -13 and 1000 mg/m2 on days -6, +1, and +8) as described previously. Patients in both groups also received thymoglobulin 1 mg/kg on days -2 and -1 if the transplant was from matched unrelated donor (MUD). In addition, the MA group received bortezomib (1 mg/m2 per dose) on days -13, -6, -1, and +2. Because the treatment groups were not randomized, we performed inverse probability weighting (Robins JM. Epidemiol. 2000; 11:550-60) to correct for potential bias in patients' selection for the comparison of GVHD.
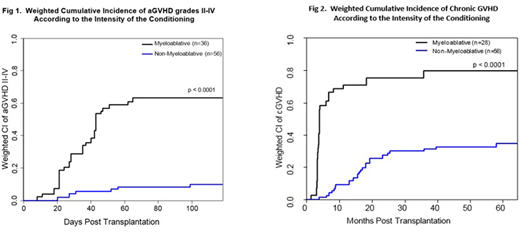
Results: The analysis included 95 patients (MA=39 and NMA=56). The majority of the patients were male (68%) with a median age of 58 years (range: 22-71). The most common histology was diffuse large B-cell lymphoma (26%), followed by chronic lymphocytic leukemia (23%), mantle cell lymphoma (19%), and follicular lymphoma (18%). Transplant characteristics included MUDs in 43 (45%) patients, 23 (24%) female-to-male donors, and 49 (52%) ABO-mismatched. In addition, CMV was reactive in 80% of patients/ and or donors. Most patients (95%) had alloSCT from peripheral blood. Significant differences between treatment groups were observed for patient age, histology, PET scan status, LDH, disease status, and prior number of chemotherapies. On average, patients receiving MA conditioning were younger (p=0.008) and had higher LDH (p=0.026) pre-transplant, compared with NMA group. In addition, a higher percentage of MA patients were diagnosed with diffuse large B-cell lymphoma (41% vs. 16%, p=0.003), had a positive PET scan (p<0.001), had refractory disease (p=0.001), and received > 3 prior number of chemotherapies (p=0.007) compared with the NMA patients. The median follow-up duration for surviving patients was 64.8 and 62.1 months for the MA and NMA groups, respectively. The groups' weighted (adjusting for patient age, donor age, donor type, disease status, sex mismatch status, histology, PET scan status, LDH, and prior number of chemotherapies) cumulative incidences of grade II-IV acute GVHD was 63% and 10%, respectively (p<0.0001) (Fig 1), grade III- IV acute GVHD 34% vs. 8% (p < 0.0001), and chronic GVHD (80% vs. 35%, p<0.0001; Fig 2), for MA and NMA groups, respectively. Among the patients who developed chronic GVHD, a significantly higher percentage of patients in the NMA group experienced de novo subtype of chronic GVHD compared with the MA group (71% vs. 32%; p=0.025) resulting in a significant difference of 5-year survival in patients who developed chronic GVHD (90% vs. 62%, respectively; p=0.047) between NMA and MA groups.
Conclusions: This is the first study to analyze the impact of the conditioning regimen intensity on the risk of GVHD in patients receiving rituximab prophylaxis with their transplants. Our results indicate that the intensity of the conditioning regimen remains a strong predictor of acute and chronic GVHD despite the use of rituximab.
Jabbour:Pfizer: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding. Oran:Celgene: Consultancy, Research Funding; ASTEX: Research Funding; AROG pharmaceuticals: Research Funding. Champlin:Otsuka: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal